If you are involved in the Aerospace, Semiconductor, R&D, Solar, or Microchip manufacturing industries – chances are you come across flammable liquids on a daily basis.
According to the National Fire Protection Association (NFPA), U.S. municipal fire departments respond to an estimated average of 160,910 fires per year that start due to the ignition of a flammable or combustible liquid; 105,520 (65%) of which started at non-residential properties.
Flammable liquids are very common, especially in our industry, and today we will be covering what are flammable liquids, how and why they can catch fire, the different classifications, and a few examples of highly used industrial flammable liquids.
Topics
[DOWNLOAD] PDF: FLAMMABLE LIQUID CATEGORIES CHART
Flammable Liquid Definition
Flammable liquids are defined by the Occupational Safety and Health Administration (OSHA) as any liquid having a closed-cup flash point at or below 200°F (93°C).
Note: The National Fire Protection Agency (NFPA) has a very different definition of a flammable liquid and even more different classification system. We break down OSHA and NFPF’s definitions in this post about flammable liquid classes and categories so there is no confusion.
What is a flash point you might ask?
The flash point of a liquid is the lowest temperature at which the liquid gives off enough vapor to be ignited at the surface of the liquid. Did you catch that? It’s actually the vapor the liquid gives off that burns, not the liquid itself, which is a common misconception.
First, let’s cover how a flash point is actually recorded.
In order to measure a flash point, scientists in a controlled environment have to introduce an ignition source to the substance, increase the substance’s temperature, and wait for the “flash”.
Once it flashes, aka: the vapor catches fire, the temperature of the substance is recorded.
There are numerous methods of measuring a flash point, but for the most part, the methods are separated into two main categories: open-cup flash points and closed-cup flash points.
Open-Cup Flash Point
In any kind of flash point test, the substance is placed into some kind of container, usually referred to as a vessel.
In the case of open-cup testing, the substance is placed in a vessel with the top open to the environment and elements.
The temperature of the substance is raised, and an ignition source is placed over the top of the vessel, waiting for ignition.
As you can imagine, this method has a lot of variables and is not quite as accurate as the closed-cup method.

Closed-Cup Flash Point
In contrast, during a closed-cup flash point test, the vessel is sealed, and the ignition source is actually inside the vessel to closer simulate real-life situations (think of flammable liquid in a drum or fuel tank).
Keep in mind that because closed-cup flash points are conducted in a controlled and sealed vessel, the flash point is likely to be at a lower temperature than during an open-cup test.
This very reason is why it is industry standard to use closed-cup flash points to ensure safer practice and handling.

Now that we have covered how we find out if a liquid is flammable, let’s discuss the different flammable liquid classifications and their different flash point ranges.
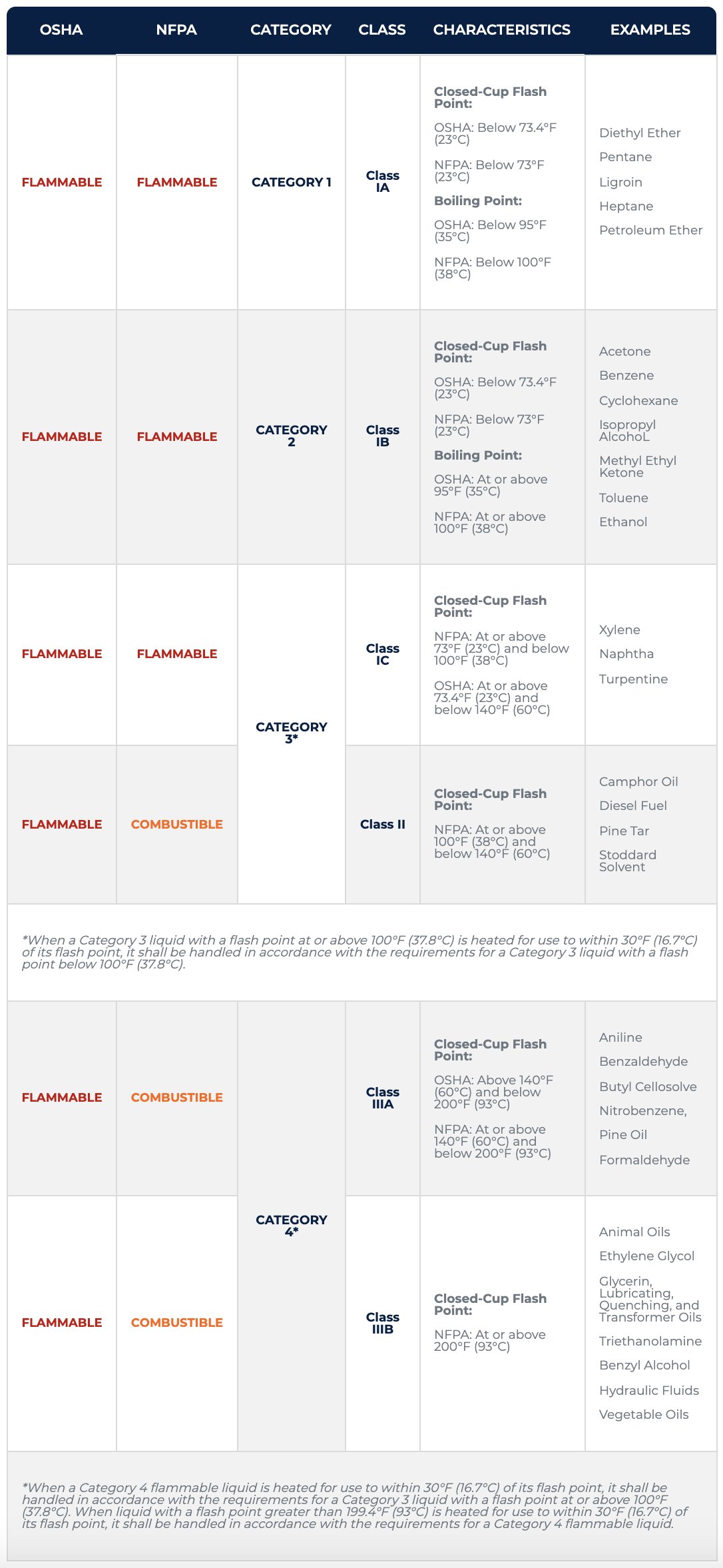
All flammable liquids fall into four distinct categories (courtesy of OSHA) based on their closed-cup flash point and their boiling point (the temperature at which the liquid starts to boil).
As you look through this chart you will also notice that there are also classes of flammable liquids.
The National Fire Protection Agency (NFPA) determines these flammable liquid classes.
As you have probably already realized – this is very confusing.
So not only did we create this great downloadable PDF of the chart below as a reference guide, but we also clear up any questions in this post >> Flammable Liquids Classes & Categories.
FLAMMABLE LIQUID CATEGORIES CHART
[CLICK TO DOWNLOAD]
As you can see, NFPA actually has a Class II, IIIA, and IIIB that are classified as “Combustible Liquids”.
According to NFPA, these would be any liquid having a flash point at or above 100°F (38°C) and below 200°F (93°C).
You can dive into the details on combustible liquids here.
So, when do we have to worry about these liquids catching on fire?
How Do Flammable Liquids Catch Fire?
When bringing up the different flammable liquid classes, we always get the question, “How would they light on fire in the real world”?
Let’s say you are a project engineer for a defense contractor in the aerospace industry. You are building a high-tech space shuttle meant to travel extreme distances in space.
Many of the materials and supplies that go into this project could include fuel, paint, solvents, cleaners, etc. – many of which are flammable liquids. You might need Xylene (a solvent), for example, to regularly clean your equipment.
Substances like Xylene have the potential to catch fire in transit – say the fuel tanker was in a highway accident, just like this truck hauling Xylene did in North Carolina on June 17, 2019.
Source: “Tractor-trailer containing flammable chemical crashes and overturns on I-40 in Greensboro, NC”, Fox News, JUNE 17, 2019, North Carolina
It can happen easier than you think.
Usually, it would take some kind of stimuli for flammable liquids to catch fire whether that be temperature or some kind of spark; however, there is something called “autoignition”.
What is Autoignition?
Autoignition is when a substance reaches a certain temperature at which it self-ignites without any obvious sources of ignition, such as a spark or flame.
Spontaneous flames? Not quite, but it is similar.
Autoignition doesn’t just happen out of nowhere.
It occurs when flammable liquids are exposed to extremely high temperatures and at that point, no spark is needed for the vapors to catch on fire.
For the most part, flammable liquids have autoignition temperatures in the range of 572°F (300°C) to 1022°F (550°C).
Think of it this way – in order to auto ignite, it would be like tossing a container of flammable liquid into a giant wildfire – extremely high temperatures and something you would never do intentionally.
However, there are a handful of substances that have a relatively low autoignition temperature.
For example, ethyl ether has an autoignition temperature of 356°F (160°C) – a temperature that every household oven can reach.
There have even been reports of ethyl ether vapors being ignited simply from hot steam pipes.
This is why the safe storage of flammable liquids is so important!
What Are Flammable Liquids Used For?
Wondering what industries actually use flammable liquids? The short answer – all.
Flammable liquids can be everyday items such as rubbing alcohol, nail polish, aerosols, even hand sanitizer.
(Now, please don’t go home and try to light your hand sanitizer on fire – very dangerous.)
Today, we want to focus on flammable liquids on a larger scale and the particular industries that use them on a daily basis.
Industries that regularly use large amounts of flammable liquids include but are not limited to:
-
- Aerospace
- Agriculture
- Construction
- Food & Beverage
- High Technology
- Industrial Plants
- Maritime
- Microchip
- Mining
- Petroleum/Oil
- Pharmaceuticals
- Transportation
- Semiconductor
- Solar
- R&D
- Telecommunications
- Waste Management
- Every industry…
It is amazing just how many materials and products go into creating the “final shelf products” that we use every day, and flammable liquids are absolutely one of those production materials.
Now that we know all about how flammable liquids are used and where, let’s take a look at some examples of the more widely used industrial-scale flammable liquids.
Examples of Commonly Used Flammable Liquids
This list is by no means a complete list of all large-scale flammable liquids, but it does include some of the most commonly used flammable substances.
LIST OF FLAMMABLE LIQUID EXAMPLES
Acetone: Category 2, Class IB
Aniline: Category 4, Class IIIA
Animal Oils: Category 4, Class IIIB
Benzaldehyde: Category 4, Class IIIA
Benzene: Category 2, Class IB
Benzyl Alcohol: Category 4, Class IIIB
Butyl Cellosolve: Category 4, Class IIIA
Camphor Oil: Category 3, Class II
Cyclohexane: Category 2, Class IB
Diesel Fuel: Category 3, Class II
Diethyl Ether: Category 1, Class IA
Ethanol: Category 2, Class IB
Ethylene Glycol: Category 4, Class IIIB
Formaldehyde: Category 4, Class IIIA
Glycerin: Category 4, Class IIIB
Heptane: Category 1, Class IA
Hydraulic Fluids: Category 4, Class IIIB
Isopropyl Alcohol: Category 2, Class IB
Ligroin: Category 1, Class IA
Lubricating, Quenching, and Transformer Oils: Category 4, Class IIIB
Methyl Ethyl Ketone: Category 2, Class IB
Naphtha: Category 3, Class IC
Nitrobenzene: Category 4, Class IIIA
Pentane: Category 1, Class IA
Petroleum Ether: Category 1, Class IA
Pine Oil: Category 4, Class IIIA
Pine Tar: Category 3, Class II
Stoddard Solvent: Category 3, Class II
Toluene: Category 2, Class IB
Triethanolamine: Category 4, Class IIIB
Turpentine: Category 3, Class IC
Vegetable Oils: Category 4, Class IIIB
Xylene: Category 3, Class IC
Can You Identify What Flammable Liquids Are in Your Facility Now?
Keep in mind that flammable liquids include any substance with a flash point at or below 200°F (93°C), according to OSHA.
It is also important to know exactly what classes of flammable liquids you have in your facilities as they have different handling and storage procedures.
You don’t want any accidental autoignition – or accidents period – happening on-site.
Can you identify what flammable liquids are in your facility now?
If you have any of the above flammable liquids in your facilities it is very important that you are aware of them and absolutely follow all of the OSHA and NFPA guidelines for handling, storage, and transportation, not only to remain audit complaint but also for you and your employees’ safety and good health.
As a quick reference, feel free to print out our chart that breaks down the flammable liquid categories and classes.
[DOWNLOAD] PDF: FLAMMABLE LIQUID CATEGORIES CHART
Disclaimer:
All content on this website is for informational purposes only. This information should not be considered complete, up to date, and is not intended to be used in place of the consultation or advice of a legal, medical, or any other professional. Chemical Strategies, Inc. assumes no responsibility or liability for any errors or omissions in the content of this site. We do not make any warranties about the completeness, reliability, and accuracy of this information. You use all information at your own risk.